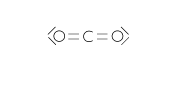The Condom - its ups and downs
Basic module Environmental Science - Groupe one
- Leen Dresen (Supervisor)
- Coen Emmer (Political Science)
- Joost van Marrewijk (Russian Studies)
- Pien Metaal (Political Science)
- Jutta Passarge (Geo-Ecology)
- David Tempelman (Philosophy)
- Annegret Thieken (Geo-Ecology)
- Christian Verberne (Political Science)
Nothing in this report may be copied or reproduced for distribution or commercial purposes without the permission of the author.
Foreword
Here is the group report of Group One, the result of an assignment for the basic module Environmental Science organised by the IVAM (Interfacultaire Vakgroep Milieukunde/ Interfaculty Departmental Group Environmental Science) of the University of Amsterdam. We were given the task of making a life-cycle analysis of a product in order to describe its possible environmental effects. This report is the result of cooperation between members of the group, the supervisor and the various sources and individuals who were approached for information.
Introduction
After extensive discussion the group chose the condom as the subject of the assignment. A number of considerations played a role here. First, as far as we knew there had been no research done on the environmental effects of condoms. During the collection of information we discovered an article that had yet to be published on this very subject and it stimulated us to investigate further into the impact of condoms on the environment (18). Another important consideration in determining our choice was the tension between the environmental effects of condoms and the fact that they are irreplaceable. Currently there is a positive attitude towards the product and its use is being encouraged to prevent sexually transmitted infections and as a contraceptive in the battle against population growth. Both these aspects are explored in the report.
We confine ourselves in this report to condoms made from natural rubber. In following the condom production chain a number of detrimental environmental effects come to light. Possible solutions that have a less negative impact on the environment are suggested in the subsequent chapters. We are acutely aware of the constraints we faced in terms of time and access to information and, therefore, of the relative value of the suggested alternatives.
One producer was chosen as a target group - the NVSH (Nederlandse Vereniging van Sexuele Hervorming/ The Dutch Society for Sexual Reform). If we proceed from a source-orientated approach to environmental problems, possible suggestions could make a direct contribution to reducing the negative environmental impact of condoms. In addition the NVSH fulfils an important social function in promoting knowledge and understanding and as such it might be expected that it would endorse the importance of environmentally friendly production and use.
In the life cycle analysis of the condom in Chapter 2, the in-use phase required by the assignment has been omitted. The reason for this was that the way condoms are used could not be described given the proposed questions. We have chosen to explore two aspects of use: the effect of use on public health and the population growth discussion (in Chapters 3 & 4). The relationship between these themes and the environmental problem is the desired increase in use and thus a growth in production and the environmental problems this brings with it. This report begins with a short historical sketch of the development of the condom and - following the conclusion - the reactions of the target group.
Research methodology
After the subject had been chosen it was necessary to allocate tasks. We decided to form small groups of two people who would be responsible for the different parts of the study. Each group collected information from the available literature as well as from companies and other organisations. This information was exchanged and discussed within the group as a whole. We are extremely aware of the constraints we faced in terms of time and access to information. In addition not all the sources we approached were prepared or capable of giving a reaction in such a short space of time, partly because of the negative publicity surrounding the subject (18). The concept that eventually emerged was critically discussed by the department. By making changes in the composition of the groups we hoped to improve the consistency of the report and improve its content.
References to literature used is indicated by the numbers in brackets given in the text. A letter refers to a person or organisation listed as having been approached for information. In addition an explanatory list about a number of chemical substances has been drawn up in the interests of clarity.
1. Historical background
Methods of avoiding pregnancy and preventing sexually transmitted infections have been in existence since ancient times. These included withdrawal, periodic abstention, vaginal douches and sponges as well as a sort of condom. The Egyptians appear to have used a condom-like linen sack that also provided protection against insect harassment and tropical diseases (5 p4). The history of the condom in Europe really begins in the sixteenth century when the sexually transmitted disease syphilis began to take on epidemic proportions. In 1564, the Italian doctor Gabriẻl Fallopio wrote that a linen sack, drenched in a solution of salt and herbs, could protect against infection (5 p4). In the eighteenth century silk and linen condoms were used as well as the appendix and bladders of lambs and goats. These skins could be used several times (5 p5). From 1839, when Charles Goodyear discovered vulcanisation, rubber entered the scene. The rubber condom could be stretched in contrast to condoms made of sheep gut which tore easily (5 p5). The introduction of fluid latex in the mid-1930s made it possible to make seamless and stronger condoms. In addition, the problem of rapid aging that affected rubber quality was solved (31, p227). Recent developments have made it possible to substitute natural rubber with artificial material. The polyurethane female condom is already on the market and a polyurethane condom for men is expected shortly. In 1949, the first coloured condoms were introduced by a Japanese company. Later came the first lubricants and spermicides (31, p227). The extent to which the development of the condom has had negative consequences for the environment is discussed below.
2. The condom: a life cycle analysis
The life history of the condom as we know it now begins with the rubber tree (hevea brasiliensis). This provides the basic material from which natural rubber is derived - latex. We define the product as latex up until the moment of vulcanisation. From that point it changes into rubber. (We have retained the term rubber tapper, however). Since the end of the nineteenth century the rubber tree has been commercially exploited. First, latex was tapped in its homeland the Brazilian forests where rubber trees grow wild (wild latex). Later plantations were developed. These are to be found primarily in Asia with Malaysia, Indonesia and Thailand producing two-thirds of the world’s supply of latex. Other developing countries - namely India, Sri Lanka and Brazil - provide most of the remainder. It is estimated that total world latex production is 5.3 million tons (12). Currently, there appears to be a surplus of latex with production outstripping consumption. In December 1991 there were reserves amounting to 1.5 million tons (12). The price of natural latex has dropped considerably in recent years because it is economically extremely dependent on the automobile industry, an industry that has itself been in stagnation.
2.1. Primairy production
2.1.1.Latex extraction
Latex extraction is a labour intensive process. Rubber tappers use special knives to cut pieces of bark from the tree. This enables the latex to trickle out of the tree and be captured. Much of the present production comes from small, independent farmers but there appears to be a tendency towards large-scale production [30].
In order to develop plantations, primary rain forest is being – and will continue to be – cut down. Often the timber felled in this way is burned immediately, devastating the natural balance of the rainforest and causing enormous CO2 emissions. After some years, the planting of young rubber trees provides the soil with a degree of protection against erosion, but the original biodiversity will have been lost forever [7].
Insecticide is not needed in rubber tree cultivation because latex provides the trees with a form of natural protection. Various methods are used to ensure that there is no undesirable undergrowth and to heighten the proactive capacity of the soil. Hand weeding is one, but it is labour intensive and therefore costly. Poison is cheaper and can be better targeted. Many of the poisons used – including sodium arsenicum and diluted diesel oil – are extremely harmful to the environment. The spot-killing method, which involves spraying only at specific locations, is common when organic and more labour intensive methods cannot be used. In large-scale enterprises, labour saving machines and fertilisers are used to maintain plantations and facilitate latex collection [29].
2.1.2. Processing
It is necessary to process the extracted latex in order to get the raw material needed for condom production. Latex cannot be used in its pure form. Processing takes place in the rubber producing countries themselves. There the objective is to limit the volume of rubber mixture and make it suitable for the further manufacture of products. Limiting the volume reduces transport costs. Condoms require a 60% latex solution and this must be of a type that has a low ammonium content (LA) to which TMDT and zinc oxide have been added (TZ).
On the plantation - immediately after extraction - an anti-coagulant is added to prevent the bacterial growth that could cause rapid thickening. An ammonium solution is used for this as well as a sodium sulphite or formalin solution (respectively 0.02, 0.04 and 0.03% per weight) [30 p462]. The accumulated mass is then transported to the factory, deposited in bulk tanks and subsequently centrifuged. The 60% solution is achieved by double centrifuge. First, the latex is inspected to ensure that the magnesium context is not too high, a factor that is important for further processing. To get the necessary low levels, diammonium hydrogen phosphate is added. As a result salt crystals are formed (ammonium magnesium phosphate) and these remain in the tanks. When the tanks are cleaned these crystals disappear into the sewage system. The centrifuges rotate for a number of hours and separate the latex mass into two parts. The latex concentrate is centrifuged again and then passed through tanks where conservatives are added. For the LA TZ type this is ammonia (0.2%), tetramethylthiuram-0-disulphide (TMTD) (0.013%), zinc oxide (0.013%) and laurate acid (0.05%) [30, p489].
It is known that TMTD can form nitrosamines that remain in the latex mass and thus later in the condom (V). Most of the ammonia will evaporate. The concentrate is transferred to storage tanks and the mixture is stirred continuously. Throughout the whole process testing takes place to maintain a perfect composition. If necessary, anti-coagulants are added.
The latex solution gained in this way is usually transported by ship to the condom factories. These are spread throughout the world and can also be found in the latex producing countries themselves.
2.2. Secondary production
The latex is transported as bulk cargo in tankers with tanks that can hold between 9000 and 14,000 litres or in 200 kilo barrels. In the condom factory the bulk latex has to be kept at a temperature of between 10°C and 15°C and is stored in stainless steel storage tanks that are equipped with turbines so that the latex can be stirred from time to time. These turbines must be able to run at 150 rotations per minute [11]. The immersion tanks are also made of stainless steel and have a filter that can eliminate those coarser particles that have a negative effect on the quality of the latex. For the same reason these tanks must be housed in dust free surroundings. This is achieved by extracting and filtering the air.
At the beginning of the production process a number of substances are added to the latex mass in the form of a water solution (see Table 1): sulphur, that is essential for vulcanisation; chemicals to speed up vulcanisation (zinc dibutyldithiocarbamate and zinc 2-mercaptobenzothiazole); emulsifiers; zinc oxide; antioxidant (2246) to slow down the aging process and, if necessary, pigment to provide any desired colour. In this way the entire mass only contains between 40% and 50% natural latex: the rest consists primarily of water.
In order to get the desired quality it is important to keep viscosity in balance. This can be achieved by keeping the temperature at a constant level with the help of a double wall that allows water to be held at the right temperature.
The condom formers are made from porcelain, glass, stainless steel, chrome-plated aluminium or soft steel. They are guided by hydraulic mechanisms when they are dipped in the latex mass to ensure constant and shock-free movement [11]. Given the fact that condoms are thin (varying in thickness from 0.03 to 0.08mm) great accuracy is required during manufacture in order to get a consistent thickness and to avoid any holes or imperfections. Normally two straight dips are carried out. After being dipped the formers are brought out of the mix in a vertical direction extremely slowly and uniformly (at the same rate as the latex flow). The latex that remains on the formers is dried by letting them rotate and then putting them in an oven at a temperature of between 60°C and 80°C for a short time. After the second dipping, the formers are again dried and guided along a line of rotating brushes [11].
The latex mix is then vulcanised in a hot air oven at between 110°C and 150°C for 20 minutes. It is only now that you can speak of rubber! The sulphur bridges that ensure strength and elasticity can no longer be undone so that defective condoms (less than 1%) cannot be reused [V]
| SUBSTANCE | DRY* | WET* |
|---|---|---|
| 60% NR latex, LA-TZ | 167,0 | 167,0 |
| 10% potassium hydroxide solution | 2,0 | 2,0 |
| 20% potassium laurate solution | 0,5 | 10,0 |
| 50% sulphur dispersion | 1,8 | 1,5 |
| 50% zinc oxide dispersion | 0,5 | 0,5 |
| 50% zinc dibutyldithiocarbamate dispersion | 1,0 | 1,5 |
| 50% zinc 2-mercaptobenzothiazole dispersion | 2,5 | 1,0 |
| 50% antioxidant dispersion | 1,0 | 1,0 |
| Water | 24,0 | 15,0 |
Pre-vulcanisation: 36 hours @ 25°C; 4 hours @ 55°C
Vulcanisation: 20 min @ 110°C to 150°C
*stripped parts by weight
After vulcanisation the rubber is cooled down. The condoms are then rinsed with water (leaching). This can be done when the condom is on the former or when it has been stripped from the former [11]. During this process some of the organic zinc compounds and other unnecessary materials are released. These substances can – via the water system or sewage sludge – end up spread over agricultural land or in depots for sludge that is polluted with heavy metals. While zinc is an essential trace element for plants, humans and animals, it is damaging in high concentrations (for some plants from a concentration of 0.2g Zn/kg. dry matter [22 p286]).
Thin rubber articles such as condoms, balloons or gloves have to be treated before stripping with either a dusting powder (such as starch – see Chapter 3) or silicon oil to prevent the surface becoming sticky. Stripping can be done dry with rotating brushes or wet with water and air from a high pressure hose.
Subsequently the formers are cleaned with toluene, a solvent. Toluene is used because it dissolves rubber easily and also gets rid of grease. It only takes a small amount of impurity to make a hole in a condom. Generally speaking, toluene should have evaporated before the former is re-immersed in the latex bath [V] This way of working can be seen as wastage (toluene being a product derived from mineral oil) and results in dispersion. It also contributes to poor working conditions.
In principle, each condom must subsequently be tested for manufacturing faults. There are electrical techniques to do this as well as inflation and water-filling tests. According to Dutch standards a condom must be able to hold 20 litres of air [6]. Currently, there are also tests being carried out using laser beams.
Once the condoms have been manufactured, they can - as far as Durex is concerned - be transported by lorry to the Netherlands. In Leerdam various substances are added to the different types of condom - spermicides (mostly nonoxynol-9), lubricants (for example, triethanolamine) [5] and substances such as benzocaine (to reduce sensitivity). Each condom is then packed in a matrix pack of plastic and aluminium foil (personal observation) to make it airtight and protect it against light. It is then distributed.
2.3 Raw materials
Unfortunately we are unable to say anything about the amount of energy used because this depends on the form of transport and on efficient energy use (the heat-power relationship). Given this, the amount of energy required for the production of different brands can vary by a factor of 5 [V].
As far as raw materials are concerned it should be noted that latex is a raw material that can be used again, but this is not so for the raw materials needed for the machinery, tanks, packaging and additives. At least with respect to the latter it is often petroleum that is the non-renewable raw material. Unfortunately we do not know a great deal about its production. However, it is to be expected that this does involve extensive environmental problems.
2.4 Disposal
After it has been used – and it is used just once – the condom can be disposed of in three ways: it can be flushed down the toilet, disposed of with other household waste or simply thrown away in the open air.
When condoms get into the sewage system – which probably happens frequently – they can cause blockages if they become entangled in the plate liners of the sewage pumps [F]. In addition, the hazardous materials in the condom go directly into the sewage water. The special grates that are intended to keep larger pieces of rubbish out of the sewage water are not fine enough to catch the condoms. As a result they get into the sludge fermentation process. The processed sludge is dried after a warm or cold fermentation process. In former times it used to be spread on agricultural land but because of pollution it now has to be taken to a waste disposal site.
According to information received over the telephone from the Dienst Riolering en Waterhuishouding Amsterdam (municipal sewage and water management service) [F] an additional problem is that the thicker rubber condom ring (the edge) is not broken down during the sludge fermentation process. As a result, once every seven years these rings have to be removed from the sludge fermentation barrels and dumped.
When the condom is disposed of as household waste or is left in the open air, it disintegrates as a result of UV light and oxygen (oxidisation) [V]. Latex can be broken down through microbiological processes [23, p418]; but we do not know to what degree this is true for rubber and/or the additives. Maybe additives such as spermicides, zinc compounds and antioxidants also affect the disintegration process.
When the condom is disposed of with household waste it may be incinerated in a waste disposal furnace. This method of processing waste generates energy in the form of steam or electricity. However, it should be noted as a negative environmental effect that while SO2, CO2 and NOx are purified, it is not possible to avoid CO2 contributing to the build-up of greenhouse gases. In addition, slag is formed that has to be deposed of in a C2-dump [D].
Because rubber breaks down relatively easily this is only a limited problem in contrast to the problems associated with other products. For example, the blockage problems mentioned earlier. It is theoretically possible to complete the raw material cycle by processing used condoms into rubber bands. However, we will not go into the practical feasibilities of this here.
2.5 Most important environmental problems
The environmental problems that arise during the life cycle of the condoms have been discussed above. The most important environmental problems it seems to us occur during the stage of primary production: the cutting down of tropical forests; the use of herbicides and the emissions that occur when raw latex is being processed. The emissions and herbicides have a tremendous local impact: they get into the catchments and sub-catchments of rivers, the soil and groundwater. In this way local supplies of drinking water are seriously endangered, not to speak of the consequences for the ecosystem and the stench. Diesel oil, to give an example of one substance, can be broken down through microorganisms, but in the natural environment this takes a very long time and can only take place if there is oxygen present [23, p418]. In addition, diesel oil can sink very quickly through the thin humus layer and into the groundwater system. When raw latex is processed into natural rubber, several chemical substances are released and these are dispersed in a variety of ways. First, ammonia which during and after being added to the latex disappears in gas form. Ammonia is an important cause of acidification.
The environmental problems that arise during the life cycle of the condoms have been discussed above. The most important environmental problems it seems to us occur during the stage of primary production: the cutting down of tropical forests; the use of herbicides and the emissions that occur when raw latex is being processed. The emissions and herbicides have a tremendous local impact: they get into the catchments and sub-catchments of rivers, the soil and groundwater. In this way local supplies of drinking water are seriously endangered, not to speak of the consequences for the ecosystem and the stench. Diesel oil, to give an example of one substance, can be broken down through microorganisms, but in the natural environment this takes a very long time and can only take place if there is oxygen present [23, p418]. In addition, diesel oil can sink very quickly through the thin humus layer and into the groundwater system. When raw latex is processed into natural rubber, several chemical substances are released and these are dispersed in a variety of ways. First, ammonia which during and after being added to the latex disappears in gas form. Ammonia is an important cause of acidification.
2.6 Alternatives
There are, however, responsible ways of producing rubber. First, primary forest should not be felled for plantation development. Further, it is possible to harvest latex without seriously disturbing the ecosystem, for example, by inter-cropping. This method involves planting rubber trees in combination with other crops such as coconut, palm oil, nut and resin producing trees. In this way the natural characteristics of the plants are used so that less herbicide is needed. Another way of reducing the amount of herbicide required is to allow cattle to graze on the undesired undergrowth. Finally, the re-evaluation of labour, leading to the use of manpower instead of chemicals and machines.
In addition, emissions that occur during processing can be easily dealt with in an effluent purification installation. Even phosphate can be removed chemically if iron [Fe3+) is added and this can significantly reduce the pressure on local river systems. However, in order to achieve these objectives there must be strict local environmental laws or else producers have to take responsibility themselves. Producers can, for example, spread effluent instead of artificial fertilisers over the plantations, and in some cases this is already happening [30].
Public health debate
The great benefits of condoms as relatively safe and also inexpensive contraceptives (see Chapter 4) and as the best possible protection against infection with STIs such as AIDS seem to make the medical risks associated with their use of secondary importance. Allergies can easily be prevented by avoiding the use of antigenic substances. But the inflammations and blockages caused by dusting powders as well as possible cases of cancer caused by additives (although relatively uncommon) could be fatal so cannot be ignored. (Evaluation of the effects of these dusting powders depends very much on the definition of 'environmental problems' that is chosen. They are natural substances that could only have a harmful effect on humans because they are used by humans during condom manufacture.)
An interesting fact is that the pathological consequences of the use of condoms – with the exception of allergies to pure rubber – are all the result of additives and dusting powders. After an extensive study of the pathological effects of additives, stopping or substantially reducing the use of additives, and the total avoidance of hazardous substances (including dusting powders, which can, for example, be replaced by silicon oil), the condoms could be unreservedly recommended from a public health perspective.
Population growth debate
We believe that there is a certain tension between population growth and environmental problems as far as condom manufacturing is concerned which we would like to discuss here. Population growth will exert such pressure on natural resources that they will be rapidly depleted [28]. DA direct comparison of the environmental problems caused by population growth with those caused by condom manufacturing make it immediately obvious that the former are more serious than the latter. The full picture, however, is more complicated. Population growth cannot be halted by increased condom use alone. Socio-cultural and religious barriers, a lack of information, and economic factors all hinder condom use.
The greatest number of children are born in developing countries. Between 1959 and 1985 the population there grew from 1.7 billion to 3.7 billion in comparison with the population of developed countries which grew from 0.8 to 1.2 billion [19, p.9]. Although the birth rate is decreasing - with the exception of sub-Saharan Africa - [25, p55], it is still high in developing countries. In general, however, population growth is the result of a worldwide fall in mortality rates.
To make things even more complicated, using condoms not only contributes to falling birth rates, but also has an effect on the mortality rate. By using a condom, the HIV virus has less chance of spreading [20], so that mortality rates fall and population growth increases.
Problems caused by population growth, such as increased deforestation to make room for farming, have encouraged national governments – partly at the request of developed countries – to set up family planning programmes. IIncluded in these programmes is the distribution of relatively safe, cheap, sometimes even free condoms. How successful the programmes are in terms of falling birth rates, will depend very much on the country or region concerned [17, p.87].
Socio-cultural and religious barriers and economic factors can be a problem when it comes to condom use. In Uganda, for example, condoms are freely distributed, but not generally used. In Ugandan society, women are subordinate to men. A woman is obliged to agree to sex, even if she knows that her husband is HIV positive. In the west of the country, a recently married woman has to go to bed with all the men in the village who contributed to her dowry. Men generally think that a condom makes them less of a man. And the Pope went on Ugandan television and argued strongly in favour of chastity and against the use of condoms [26]. Finally, as far as economic circumstances are concerned, the rule in poorer countries is that more children mean more hands and more income [19].
A wider distribution of condoms does not automatically lead to a decline in population growth. It is therefore difficult to justify the assumption made earlier in this section.
Conclusion
Nothing can take the place of the condom. Increased use of condoms is advisable as protection against AIDS and as an inexpensive contraceptive. We do not consider the latest developments in the condom industry - replacing rubber with polyurethane (PUR) – as desirable. Particular because PUR is made from a non-renewable raw material (petroleum). Using latex and rubber is unavoidable, but we believe that it is possible to make the production process cleaner than it has been up until now.
Primary production can take place in a responsible manner. The prerequisites for this are:
Primary forest should not be felled for plantation development;
There should be no large-scale plantations (monocultures);
Emissions during raw latex processing should be avoided, particularly into rivers.
Transport (per ship) will still be necessary. Hardly any rubber is being grown in Europe at the moment. Portugal seems to be a possible alternative for the future (although there are opposing views on this) [5].
We note the following with regard to secondary production: internal recycling is impossible; the addition of sulphur is essential; other additives (zinc compounds) also seem to be unavoidable but are certainly harmful. Policy should therefore be directed towards the avoidance of emissions, in combination with good housekeeping.
With regard to public health, the only problems seem to be additives and dusting powders. If the use of additives were reduced and no more hazardous substances such as dusting powders were used (also better from an environmental point of view, as these substances are produced in chemical factories) condoms would be no problem at all from a public health perspective.
Disposal is unavoidable; recycling is of very little benefit to the environment and it is not very feasible from a practical point of view. Disposal should, however, be done in a responsible manner, as the condom does contain hazardous substances. We think disposal advice should be given on the packaging itself.
Target group
The target group we chose was the NVSH (Dutch Society for Sexual Reform), because this organisation has two different functions. It has a brand of condoms on the market and also provides the general public with information on sexuality. The latter was what made the NVSH particularly unusual and interesting for us. We thought that they, more than any other manufacturer, would want to take into account the environmental aspects of manufacturing and use.
As a manufacturer, the NVSH is represented by its sales division, the Centraal Middelen Depot Nederland BV (CMDN) in The Hague. The CMDN has a list of requirements which have to be met by its own factory and by other suppliers. These comply with the Wet Besluit Rubbercondomen (rubber condom legislation) and have an effect on the production process.
Reactions
The target group did not take action on all the points mentioned in the conclusion [S]. According to the CMDN, there had been a 25% increase in condom use in the West in recent years but this has now levelled off. A global increase is not expected. Distribution in Third World countries only has some of the desired effect due to various religious and cultural restrictions. A possible rise in latex production is more likely to be the result of a higher demand for other rubber products, such as car tyres.
The CMDN does not feel obliged to try to influence primary production with regard to environmental aspects: "The producer in the country of origin must comply with that country's environmental legislation."
The CMDN did not agree with our proposal for reducing transport distances. Soil composition and climatological conditions mean that the quality of Portuguese latex will never compare with the quality supplied by the current latex-producing countries. The NVSH has been giving disposal advice on its packaging for some time now to ensure that condoms are not flushed down the toilet (but so discreetly, that we hadn't noticed it).
Appendix
Appendix A: List of chemical substances
| Substance | Structural formula | Properties/effects |
|---|---|---|
| Ammonia (NH3) | 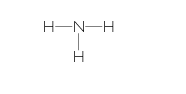 |
- Colourless gas |
|
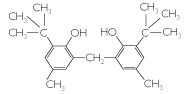
Antioxidant 2246 (2,2’-methylenebis(4-methyl-6-tert-butylphenol) |
 |
|
|
Carbon dioxide or carbonic acid gas (CO2) |
|
|
|
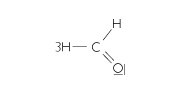
Formaldehyde (formaline, methanal) |
 |
|
|
Hydrocarbons |
|
|
|
Mica |
|
|
|
Mineral oil |
|
|
|
Nitrogen oxides (NO and NO2) |
|
|
|
Nitrosamines |
|
|
|
Nonoxynol-9 |
|
|
|
Potassium laurate and laurate acid (n-dodecanoic acid) |
|
|
|
Rubber |
|
|
|
Sulphur (S8) |
|
|
|
Sulphur dioxide (SO2) |
|
|
|
Talc |
|
|
|
Tetramethylthiuram Disulphide (TMTD) |
|
|
|
Toluene (methylbenzene) (C6H5CH3) |
|
|
|
Zinc Dibutyldithiocarbamate (ZDBC) |
|
|
|
Zinc-2-Mercaptobenzothiazole |
|
The information in the table can be found in references 1, 4, 10, 15 and 22. (This table is not intended to be exhaustive.)
Appendix B: Process Tree Diagram for rubber condoms
Download pdf "Process Tree Diagram for Rubber Condoms".
References
- Beyer & Walter (1988), Lehrbuch der organischen Chemie. 1st ed. Stuttgart.
- Bircher, A.; Hirsbrunner, P.; Langauer, S. (1993), Allergic contact dermatitis of the genitals from rubber additives in condoms. From: Contact dernatis. Vol. 28.
- Concar, D. (1993). Love me tender. New Scientist, 2 Oct. p. 43
- Copius Peereboom; Reijnders (1986), Hoe gevaarlijk zijn milieugevaarlijke stoffen? Boom Meppel, Amsterdam.
- Condomerie Het Gulden Vlies (1989), Catalogue. Amsterdam.
- Various authors (1991), Condom test, Consumentengids. October, no. 10, p. 626-629.
- Goodland, R.J.A. (1984), Environmental Management Tropical Agriculture, Chapters 11 and 12.
- IVAM (1993), Syllabus basic module environmental science, September, Amsterdam
- Kang, N.; Griffin, D.; Ellis, H. (1992), The pathological effects of glove and condom dusting powders. From: Journal of applied toxicology, Vol 12, No. 6, p. 43
- Kloeg, Daan. (1991), Natuur en Milieu, encyclopedia Zomer & Keuning boeken, Ede.
- Malaysian Rubber Producers Research Association, Natural Rubber Technical Information Sheets. 1976 no. 1, 1977 no. 15, 1977 no. 16, 1984 no. 61.
- Malaysian Rubber Review, 3rd quarter 1991.
- Miles, A. (1984). Barrier contraception. Clinical Obstetrician Gynaecology, Vol 11, No.3.
- Novello, C. (1993), US Public Health Service From the Surgeon General. JAMA, June 9, Vol. 269, No. 22.
- Nutt, A.R. (1984), Toxic Hazards of Rubber Chemicals. London: Elsevier Applied Science Publishers.
- Paine, C.G.; Smith, P. (1957), Starch granulomomata. From: Journal of Clinical Pathology. Vol 10.
- Ringhem, K. (1993), Factors that determine prevalence of use of contraceptive methods for men. From: Studies for Familyplanning. Vol 24, no. 2, p. 87- 99.
- Robles, M., Sparidaans, A. (1993), De waarheid over ekoseks, From: De Kleine Aarde. Autumn ’93 no. 86.
- Sadek, N. (1989) Safeguarding the future, UNFPA.
- .Salem, A. (1992), A condom sense approach to AIDS prevention: a historical perspective. From: Medline. October.
- Saxen, L.; Kassinen A.; Saxen, E. (1963), Peritoneal foreign body reaction caused by condom emulsion. From: Lancet, Vol 1.
- Scheffer, A. (1989), Lehrbuch der Bodenkunde. 12th ed. Stuttgart.
- Schlegel, H.G. (1985) Allgemeine Mikrobiologie. 6th ed. Stuttgart: Thieme
- Sikkenk, M. (1992), Nonoxinol-9: meer dan zaaddodend alleen? Utrecht: Wetenschapswinkel Biologie. November.
- Tellegen, E. Wolsink, M. (1992), Milieu en samenleving. Leiden/Antwerp: Stenferd Kroese.
- Temmerman, E. (1993), Afhankelijkheid maakt Oegandese weerloos tegen aids. De Volkskrant. 23/09/93.
- Turjanmaa, K.; Reunalam, T. (1989), Allergic reactions to rubber condoms. Genitourin Medicin. Vol. 65.
- Vereniging Milieudefensie. (1992), Actieplan Nederland Duurzaam. Amsterdam.
- Vonk, R. (1991), Grondstoffenspecial. From: Internationale Samenwerking. May, p.9-10.
- Webster, C; Balkwill, W.J. (1989), Rubber. London, Longman Group UK Ltd.
- Youssef, H. (1993), The history of the condom. From: Journal of the Royal Society of Medicine. April, Vol. 86.Beyer & Walter (1988), Lehrbuch der organischen Chemie. 1e druk Stuttgart.
INDIVIDUALS AND ORGANISATIONS CONSULTED DURING RESEARCH
- Ms Meeuwik, Afvalverwerking Rijnmond, the Netherlands (household waste disposal service).
- Ms de Beer, Brokatrade, the Netherlands (importer of female condoms).
- Mr CBS Ittersen, the Netherlands, provided information on the scale of condom use.
- Chemiewinkel, K.v.A., the Netherlands (chemical consultancy).
- Ricky Janssen and Theodoor van Boven, Condomerie Het Gulden Vlies, the Netherlands.
- The laboratory at Dienst Riolering en Waterhuishouding Amsterdam, the Netherlands (municipal sewage and water management service).
- Femidom female condoms advisory service, the Netherlands.
- Greenpeace, the Netherlands.
- Ms Kamp and Mr Heij, Instituut voor Kunststof en Rubber, the Netherlands (association for the plastics and rubber industries).
- Mr Lindhout, the Netherlands, provided information on the substances found in condoms.
- Ms I Zijderveld, Marketing Coordinator, London Rubber Company NV, the Netherlands.
- John Wood, Operations Director, London Rubber Company Ltd, England.
- Keuringsdienst van waren (Dutch government agency for food and consumer safety)
- .KIWA, Nieuwegein, the Netherlands (quality assurance for sanitary appliances).
- Michel Robles, Kleine Aarde, the Netherlands (centre for sustainability).
- The product safety department at the Dutch Ministry of Welfare, Public Health and Culture.
- NEN (Dutch institute for standardisation).
- Mr Julsing, Centraal middelen depot, NVSH, (the sales division of the Dutch Society for Sexual Reform).
- Mr Hoefnagel, Rubberstichting, the Netherlands (information centre for natural rubber).
- Rubber Tropen museum.
- Jaap Havinga and WJ Aben, library, TNO, the Netherlands (Dutch scientific research organisation).
- Royal Tropical Institute, the Netherlands.